Description:
The current state of the art:
Inflammation is associated with many serious diseases, for example, rheumatoid arthritis, chronic hepatitis, myocardial infarction, brain ischemic injury, cancer, and pulmonary diseases. Anti-inflammatory drugs such as non-steroidal (including ibuprofen, naproxen, indomethacin, etc) and COX2 inhibitors have been in the market for a long time but they have various side effects including gastrointestinal ulceration, bleeding nephrotoxicity and serious side effects such as heart and renal failure.
The problems with the current art:
Novel anti-inflammatory active agents with enhanced potency and fewer side effects are still in urgent need. Curcumin is a safe natural compound that exhibits many biological properties including anti-inflammatory properties. Despite the vast biological properties of curcumin, its drawbacks (low aqueous solubility, stability, and bioavailability) restrict its therapeutic applications
The advantages of our Invention:
Scientists at AU synthesized curcumin conjugates by coupling amino acids (glycine, alanine, DL-alanine, valine, leucine, methionine, phenylalanine, tryptophan,) and protecting groups (benzyloxycarbony (Cbz) and fluorenylmethyloxycarbonyl (Fmoc)) with curcumins. These conjugates have increased bioavailability and similar efficiency as curcumin in combating cancer, inflammation, and microbial growth.
In acute carrageenan-induced paw edema in rat, 10 mg/kg rat body weight of synthesized conjugates exhibit promising anti-inflammatory properties with potency higher than that of indomethacin: the percentage (%) inhibition of edema of Cbz-DL-Ala-Cur, Cbz-Met-Cur, Fmoc-Met-Cur, Z-D-Ala-Cur, Z-D-Met-Cur, Boc-L-Met-Cur, Boc-D-Met-Cur, HCL-Gly-Cur, HCL-D-Ala-Cur, HCL-β-Ala-Cur, HCL-L-Met-Cur, and HCL-D-Met-Cur relative to that of indomethacin at 4 h effect are 115, 103, 113, 115, 108, 102, 101, 110, 102, 111, 113, respectively.
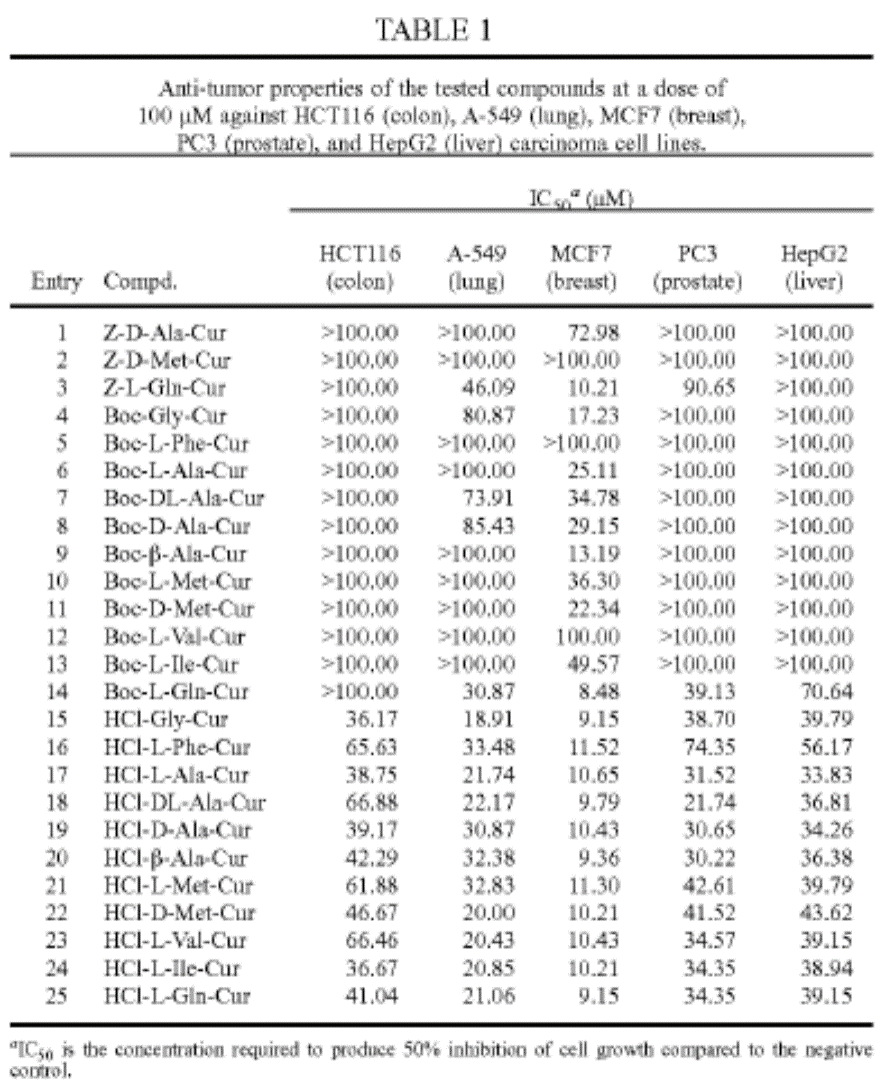
In colon (HCT116 cells), lung (A-549 cell), breast (MCM7 cell), prostate (PC3 cell), and liver (HepG2 cell) cancer cells, HCl curcumin conjugates have lower concentrations IC50 to inhibit cell growth than other conjugates. (Table 1).
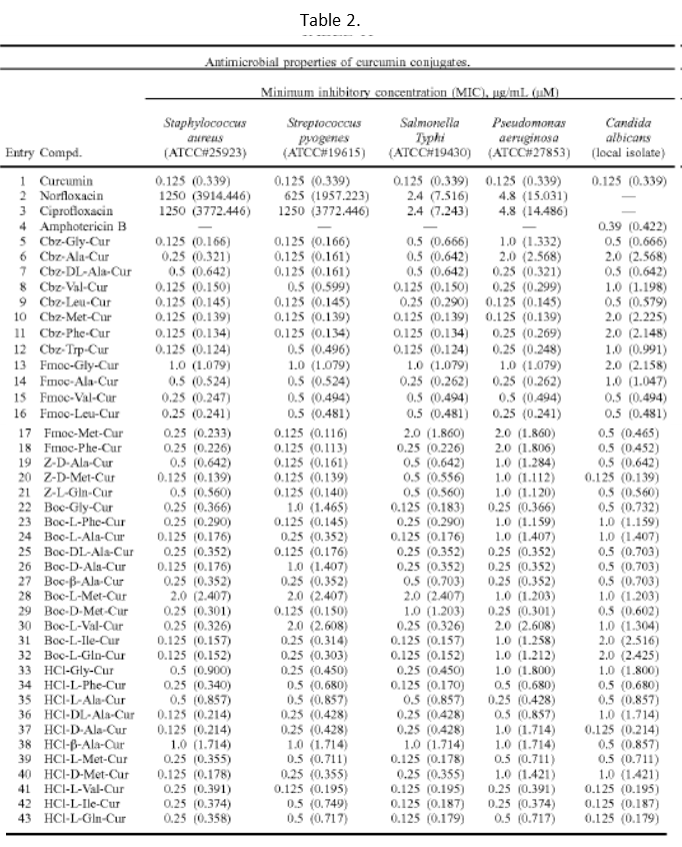
Synthesized curcumin conjugates demonstrated much lower minimum inhibitory concentrations (MICs) against the microorganism, Staphylococcus aureus, Streptococcus pyogenes, Salmonella typhi, Pseudomonas aeruginosa and Candida albicans than antibiotics (norfloxacin, ciprofloxacin, and amphotericin B) (Table 2).
When administrated orally to animals, the synthesized conjugates cause no ulcers, lesions, or erosions to the tested animal gastric mucosa revealing the safety of these conjugates for oral administration/application. Therefore, the synthesized curcumin conjugates can be a potent and effective anti-inflammatory candidate for the treatment of cancer and bacterial infection.
AURI: 2017-012
IP status: US patent 10660967
Reference: https://pubmed.ncbi.nlm.nih.gov/32311607/
PI: Siva Panda, Ph.D. https://www.augusta.edu/scimath/chemistryandphysics/siva-panda.php