Description:
The current state of the art:
Nonsteroidal anti-inflammatory drugs (NSAIDs) have broad therapeutic use that ranges from the treatment for fever, inflammation, and mild pain to severe chronic inflammatory disorders. NSAIDs mainly act by COX 1 inhibition, the key enzymes capable of the conversion of arachidonic acid to prostaglandins. Therefore, the long term use of NSAIDs may lead to severe gastrointestinal damage and renal dysfunction.
The problems of the current art:
The development of a completely selective NSAID toward COX2 is still a long-standing problem.
The advantages of our invention:
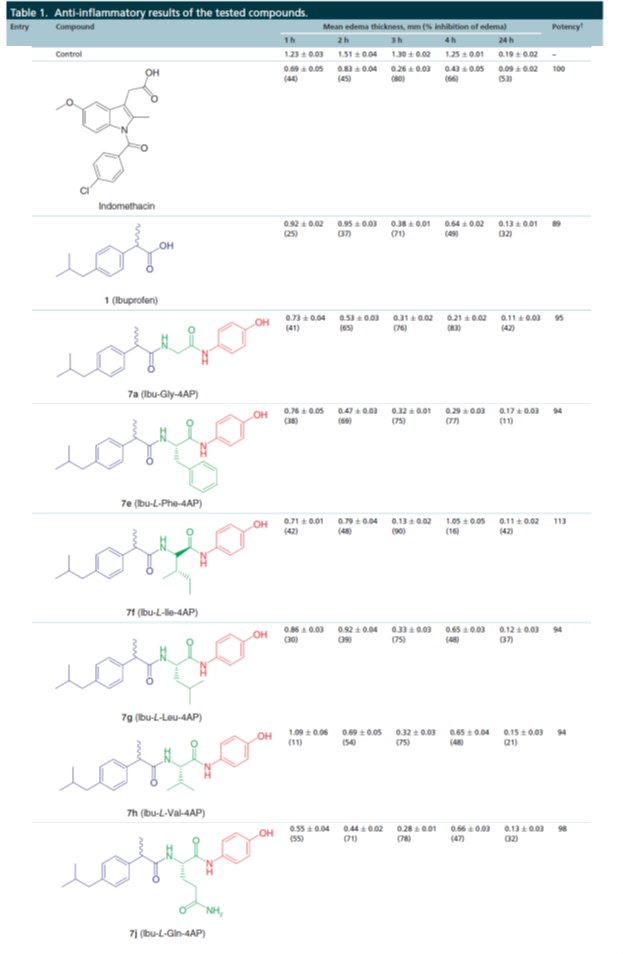
Scientists at AU developed ibuprofen conjugates using the carboxylic acid group of ibuprofen with 4-aminophenol (precursor of acetaminophen) via amino acid as a linker. The new hybrid conjugates possess potent anti-inflammatory activities in the carrageenan-induced paw edema technique (Table 1).
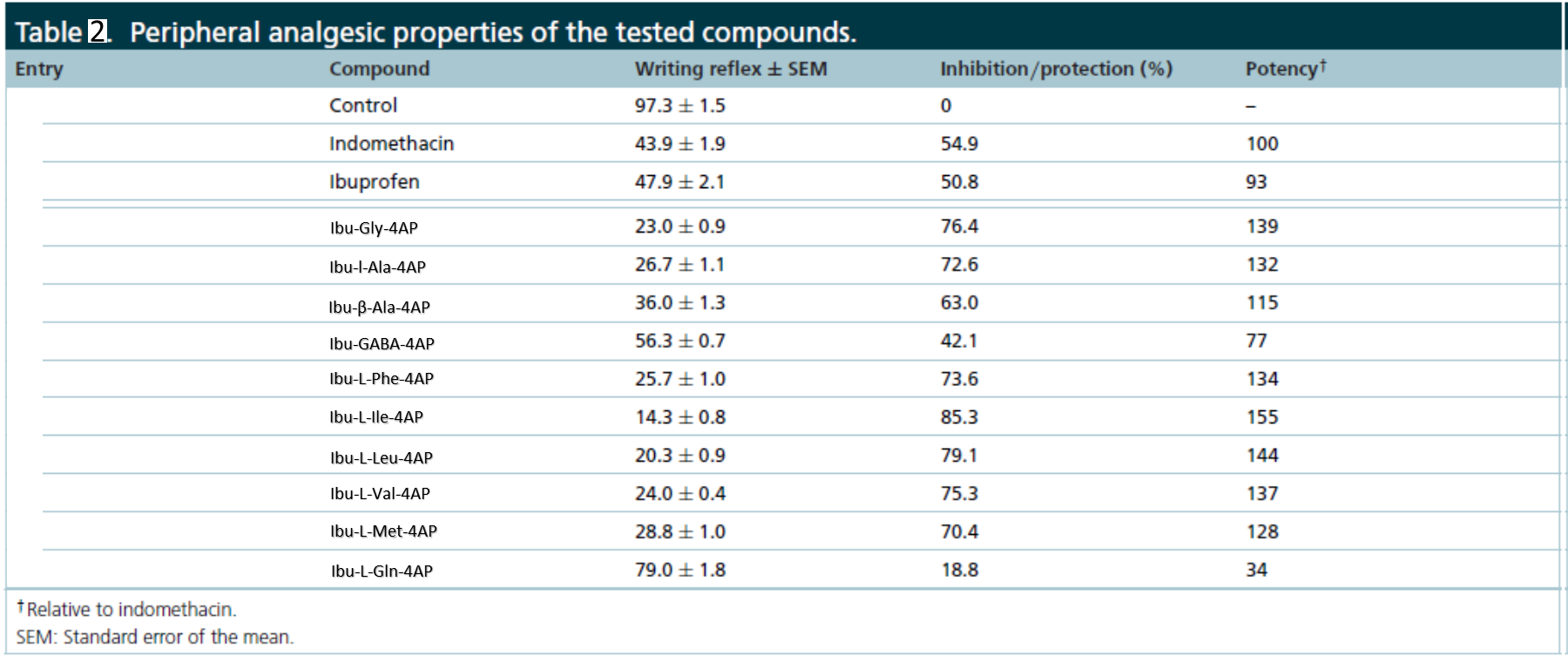
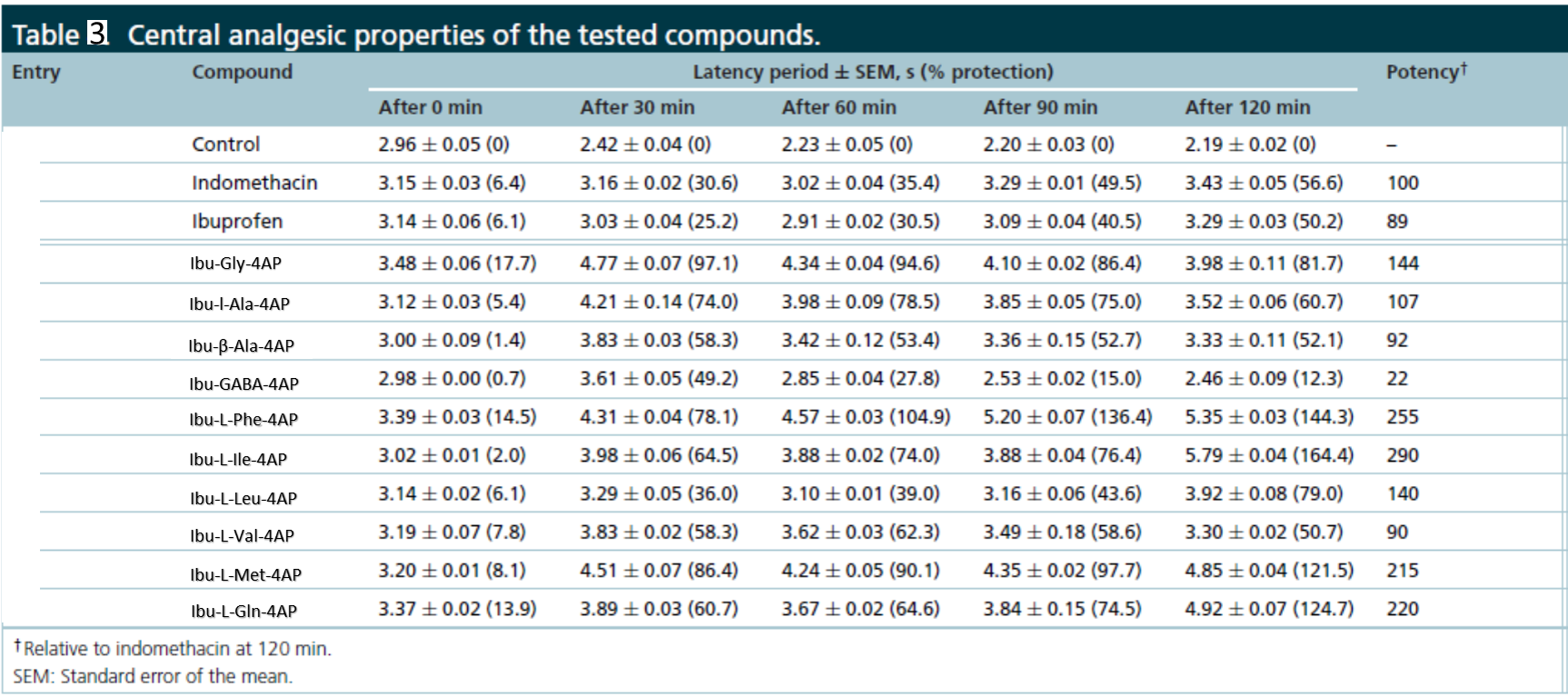
In in vivo acetic acid-induced abdominal writhing study, the hybrid conjugates enhanced peripheral analgesic properties (Table 2). In in vivo hot plate study, hybrid conjugate enhanced the central analgesic properties (Table 3).
Conjugate lbu-L-Phe-4AP selectively inhibits COX-2 compared to its parent precursor, ibuprofen in COX-1/2 bioassay. Moreover, the hybrid conjugates do not cause ulcers or lesions in the animal gastric mucosa and are safe to the animals when give fivefold and tenfold to the animals.
Therefore, the ibuprofen hybrid conjugates are promising candidates for the treatment of inflammation associated ailments, fever, and pain.
AURI # 2020-037
IP status: provisional 62/027,081
Reference: https://pubs.rsc.org/en/content/articlelanding/2019/ra/c9ra03380g#!divAbstract
Lead Inventor: Siva Panda, PhD https://www.augusta.edu/scimath/chemistryandphysics/siva-panda.php