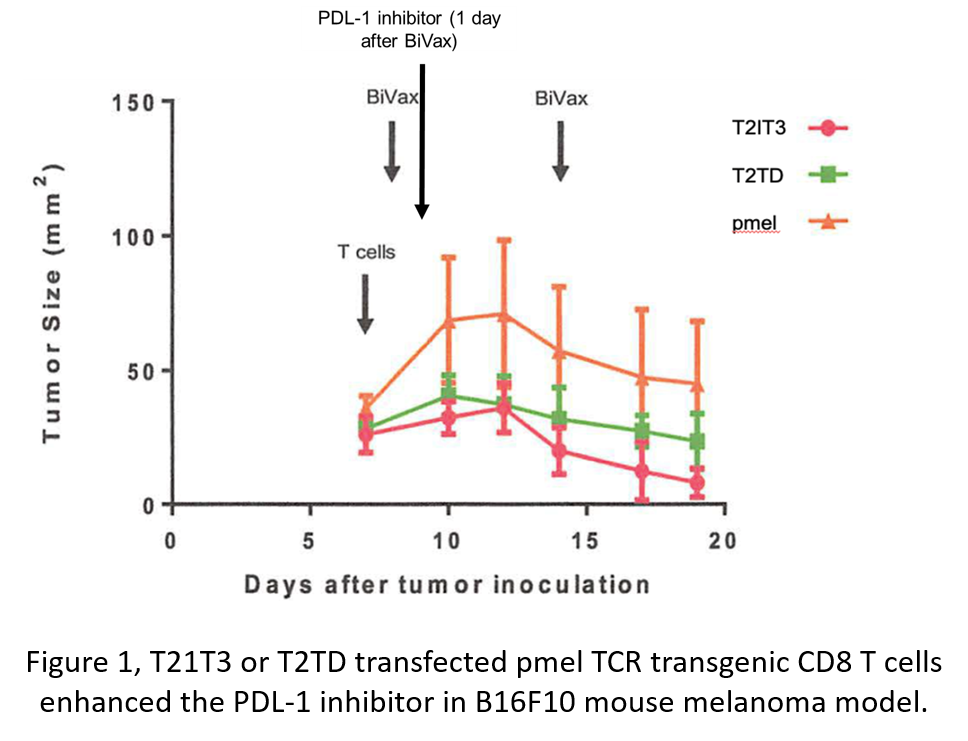
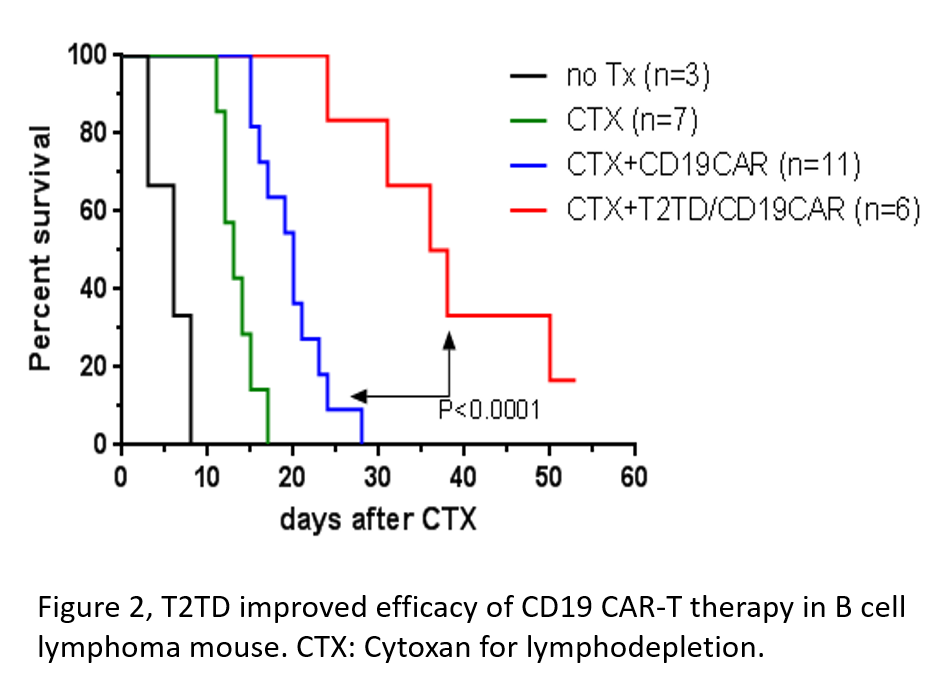
Description:
The current state of the art:
Cancer immunotherapy resurrects the patients’ suppressed immune system by increasing the immunogenicity of T cells such as the adoptive transfer of T cells (TCR and CAR T cells) and removing suppression of immune checkpoints such as PD-1/L1.
Many cancer immunotherapies have been approved by the FDA. For example, Yescarta (CAR T therapy) has been approved to treat lymphoma; Keytruda (PD-1 inhibitor) has been approved to treat melanoma, lung cancer, head and neck, Hodgkin lymphoma, and stomach cancer.
The problems of the current art:
While cancer immunotherapies are promising in both blood and solid tumors, they are more effective in blood tumors than in solid tumors. In the solid tumor, there are multiple mechanisms of immunosuppression in the microenvironment that blunt the capacity of these cells to attack or to even reach the solid tumor microenvironment in sufficient numbers. More cancer immunotherapy candidates are needed to improve drug efficiency in solid tumors.
The advantages of our invention:
The scientists at AU developed novel composition of matters and methods to reverse the immune suppression and enhance cellular therapy by simultaneously inhibiting the expression of multiple immune negative regulators through dominant-negative modulation. Two of the compositions, T2IT3 (TGFβR, IL10RA, and Tim 3) and T2TD (TGFβR, Tim 3, and DR5) enhanced the efficacy of PD-L1 inhibitor and prolonged the immunogenicity against tumors in pmel TCR transgenic CD8 T cell mouse melanoma model (Fig. 1). Moreover, composition T2TD-transfected-CD19 CAR-T cells improved tumor survival compared to CD19 CAR-T cells alone and prolonged reduction of CD19-positive peripheral blood B cells in the subcutaneous A20 lymphoma model (Fig. 2). Therefore, the current invention can be a promising candidate to enhance cancer immunotherapy in both solid and blood cancer.
AURI # 2019-019
IP status: US Provisional 63/008,335
Lead Inventor: Pandelakis Koni